But what does Agent Orange have to do with symmetric molecules?
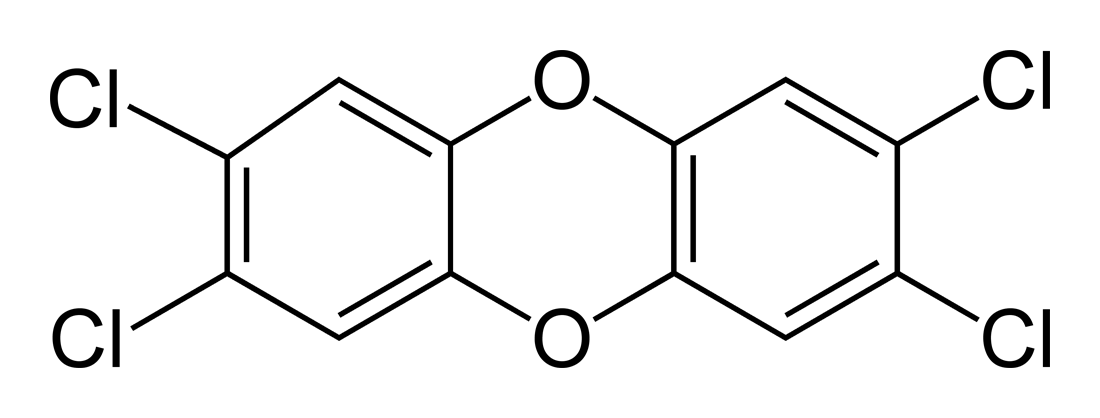
(A 2,3,7,8-TCDD molecule diagram)
Well, you can see from the molecular structure shown above that, in fact, this molecule is almost perfectly symmetric! (For those of you who are group-theory-nuts out there, this molecule possesses D2h symmetry! More to come on that later on in the blog.)
Other characteristics of its symmetry include the following:
- All atoms in the molecule have completely full octets-- in other words, there are no electrons missing from their valence shells.
- There are no net dipole moments, which can be easily seen from the symmetric nature of the molecule.
- No formal charge differences exist among the atoms.
- No angles between atoms are "strained": all bonds are in their ideal angles.
So we can see just how beautifully symmetric this molecule really is.
But what does its symmetry have to do with its side-effects?
If you think that the molecule's symmetry is just a coincidence, and that the dangerous side-effects of this molecule stem from other properties, you are not fully correct; its symmetry does affect its level of danger!
Because of the symmetry discussed above, the molecule is VERY stable, which only adds to the damage. This stability gives 2,3,7,8-TCDD a half-life of 7-12 years, which means that the toxin will last for a very long time. So not only are the side-effects of TCDD-exposure devastating, but the risks of being exposed long after its introduction are also very high.
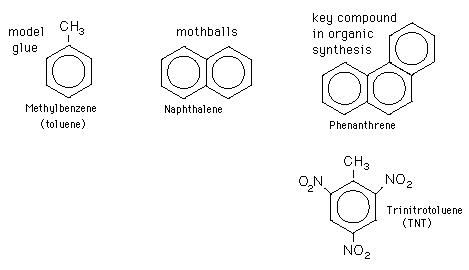
Also, because this dioxin shares structural similarities between it and aromatic hydrocarbons (or molecules made up of carbons and hydrogens and containing at least one benzene ring), the TCDD molecule can fight for the receptor site of aromatic hydrocarbon proteins. This allows the toxin to be absorbed into the body more easily.
(Examples of aromatic hydrocarbons: Each big circle and hexagon in the diagram represents a benzene ring, which is an actual "ring" composed of 6 carbon atoms and 6 hydrogen atoms.)
So overall Agent Orange shows that despite their beautiful appearance and stability, symmetric molecules can be just as dangerous as any other molecules-- and in this case, even more dangerous.
Lastly, we now have to ask ourselves-- is it really worth pursuing the formation of new chemical products or other symmetric molecules? After all, although 2,3,7,8-TCDD was a by-product, it was still human-made-- we essentially brought this upon ourselves! So is it worth playing around with such molecules, with the giant health risks on our backs? We leave this question open to the reader.