Mainly, group theory immediately answers whether any molecule can or cannot be polar or chiral.
1) Polarity:
Polarity is a subject that we touched upon in a lot of previous posts, and also something that you should be familiar with.
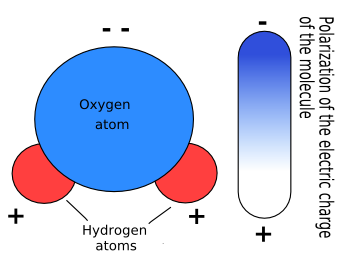
If you look at the H2O molecule above, you will notice that the oxygen atom "hogs up" the electrons within the hydrogen bonds! Thus this molecule-- water-- is polar.
In general, polar molecules will have asymmetric charge distributions-- i.e. some atoms will be more positively or negatively charged than others. This is caused by an intermolecular force called "dipole-dipole" interaction, in which the more positively charged atom(s) tend to pull the electrons closer to themselves, causing a shift in charge and a slight imbalance.
So how does this relate to group theory? Well, if we remember our point group table from before, you'll remember that there are many point groups involving the symmetric property that interchanges the two end-atoms of a molecule-- such groups include Dnd, which include a symmetry plane:
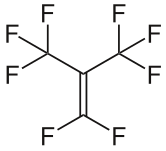
With such even symmetry, it's no surprise that molecules in that group can't have any uneven distributions of charge!
Moreover, if we look at Cn groups, we can see that the dipole moment cannot happen perpendicular to the axis of symmetry-- it's just to symmetric! For example...
If you see that one, it's just too symmetrically formed to share any dipole moments along the axis.
However, that doesn't always mean that Cn can't have any molecules with dipole moments. Take a look below:
For the specific molecule NH3 above, we can see that although there is an axis of symmetry, the molecule is still polar! Geometrically, we can see that all the hydrogens are "pointing down," causing an imbalance of charge.
So in conclusion, the following statement can be made:
"The only groups compatible with a dipole moment are Cn, Cnv and Cs."What do we mean when we say "compatible"? We mean that these are the only groups that might have dipole-dipole forces. Particularly, for molecules in Cn or Cnv, we can see that if anything, the dipole has to lie along the axis of rotation.
Next up:
2) Chirality:
Molecules that are chiral basically has a non-superimposable mirror image molecule.
What does this mean? Well, if you're scratching your head at the confusion, just look at your two hands for a second:
You can easily get from one hand to the other by reflecting it across that mirror plane...But no matter how many translation operations that you try, you won't be able to recreate the other hand.
Now imagine that with molecules...that's what we mean.
This next picture might help clarify more:
(The molecule in hand (pun intended) are enantiomers (isomeric pairs of molecules that are chiral) of a generic amino acid.)
Now here's the kicker-- group theory tells us exactly which groups of molecules can't have chiral molecules!
If we think about the symmetry operations, having any mirror planes or centers of inversion would definitely cross out the chances of having chiral molecules, right?
With an inversion symmetry, for example, we know that any "mirror" image molecule can be superimposed using inversion.
And that's not all: the improper rotation can also preclude any chiral molecules:
The picture pretty much sums up the point-- that if a molecule has improper rotation symmetry, then its chirality can also be ruled out.
Therefore, the previous statements can be summed up as the following:
Any molecule with a center of inversion or a mirror plane of any type cannot be chiral. Otherwise, as long as the molecule cannot be improperly rotated, the molecule has the chance of being chiral.
That's it for the very immediate consequences, but don't be fooled! Group theory has implications in chemistry far beyond what we just covered in this post.
So who said that math and chemistry don't mix? Clearly no one who knows about group theory!