To recap: we assign different types of symmetries with different notations so that we can differentiate one from another easily.
Last time, we talked about three of the 5 main symmetries: The identity, Rotation, and Reflection!
(Here you can see both the reflection planes and the rotation axis of H2O!)
Today we'll talk about the other two:
4) Improper rotation (Sn)
So we know about rotations..but that's an improper rotation?? Well basically, it's a combination of a rotation AND a reflection! Funky right?
As always, it's always best to look at an example:
Here is a ball-and-stick model of methane (CH4). If you look at the two different sides separated by the green plane, you'll notice that we can get to the right side by reflecting it, and then turning it a bit around the green axis! That's the essence behind the improper rotation:
An Sn improper rotation is a reflection followed by a rotation by 360/n degrees.
So in this case, this would be an S4 improper rotation!
We should note here that, while most molecules with Sn symmetry also have rotational and reflective symmetries, some don't have to! For example, allene (C3H4) has the former but neither of the latter:
You can see how it has S4 symmetry, with the axis through the three carbon atoms and the plane perpendicular to the axis and through the middle carbon. However, it does not have either σ4 or C4 symmetry.
Finally, we arrive at the last (and, to me, the most confusing) symmetry:
5) Inversion (i)
Inversion turns a molecule "inside-out." Basically, if a molecule has inversion symmetry, it has an inversion center. For example, a cube or sphere would have such a center. Similarly, benzene would also have it:
However, other molecules like water don't.
If you're confused, don't worry-- it only gets more complicated.
Having this symmetry would mean that we can arrive at the same molecule by moving each atom of the molecule along a straight line through that inversion center, to a point equally distant from the original position of the atom.
What does that even mean??? Let's find out with an example:
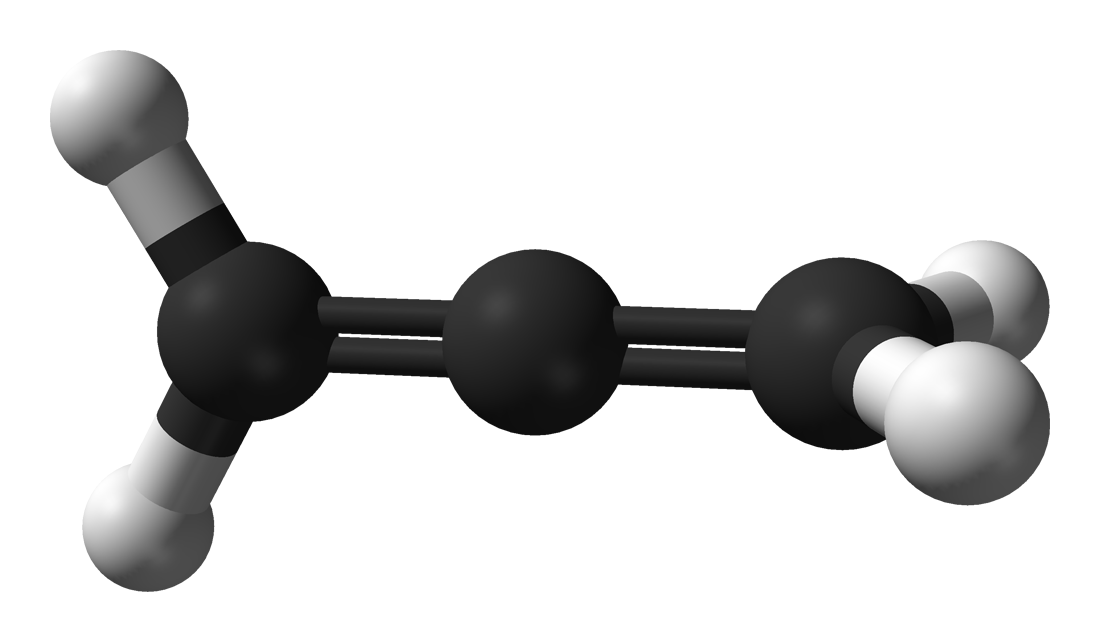
If you see to the right, the ethane molecule has an inversion center, because we can take each of the hydrogen atoms, move it in a straight line through that center, and arrive at one of the other hydrogen atoms!
If that still confuses you, don't worry-- look at the right diagram. In SF6, each fluorine atom moves in line with the center, or the sulfur molecule in this case, and ends up in the position of a different fluorine atom. That's the idea of inversion.
Here are a couple of more examples:
This is a hexacarbonylchromium complex Cr(CO)6. We can clealy see that the center is the Chromium atom, and that each carbon and hydrogen atom can be moved to another of the same molecule.
Easy enough? Well too bad. Here's one that's not so obvious:
This is a staggered 1,2-Dichloroethane (C2H4Cl2) molecule. The center is a bit more hidden in this case, but the picture shows you quite nicely. If you follow the red, dotted line, you'll see that each atom matches the other perfectly. So this has inversion symmetry.
(Also note the S2 symmetry at work here. Coincidence? We encourage the reader to explore.)
Here's where things get funky. Here is eclipsed 1,2-Dichloroethane:
Even though it has the same molecular formula, this one doesn't have the same inversion symmetry! Rather, it has reflective symmetry-- something that the staggered form doesn't have! This tells us that even though molecules may have the same formula, they may have completely different symmetries. We have to be wary of that.
So overall, these are the 5 main symmetries of a molecule: The Identity, Rotation, Reflection, Improper Rotation, and Inversion. This is summed up in the table below:
So, we've finished going over the basics..but now what? Why is this so important? What does this tell us about any properties of the molecule? How can we use this group theory, and what does one symmetry operation have to do with another? We'll continue this journey on a future post.








No comments:
Post a Comment