 |
| Symmetric "Hydrogen Bond" |
Symmetrical "hydrogen bonds" have the potential to be one of the strongest bonds, but what makes a regular "hydrogen bond" symmetrical? A study conducted at Yale University in October of 2012 by Schley involved analyzing iridium (III) alkoxides to determine what gives rise to a symmetrical "hydrogen bond". Utilizing x-ray diffraction, he found, along with his coleagues, that very short hydrogen bonds with O···O distances of 2.4 angstroms exist. After a series of calculations, Shley uncovered that a shorter O···O bond distance will give rise to a symmetrical hydrogen bond.
| Symmetrical "Hydrogen Bonds" in Iridium (III) Aloxides |
dicarboxylate monoanions. Resulting from this analysis is that the intramolecular "hydrogen bonds" are asymmetric, although can become symmetric in a crystal structure.
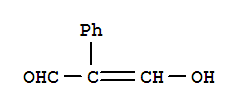 |
| 3-hydroxy-2-phenylpropenal |
Another article by Charles Perrin states that symmetrical "hydrogen bonds" can occur only in a crystal. He supports this claim by stating that a disorded environment, in solution, will produce an asymmetric bond. However, crystals, such as when water is in its solid phase existing as ice, can guarantee symmetry. So does this mean that symmetric hydrogen bonds can occur only in the solid phase, and never in the gas or liquid phase?
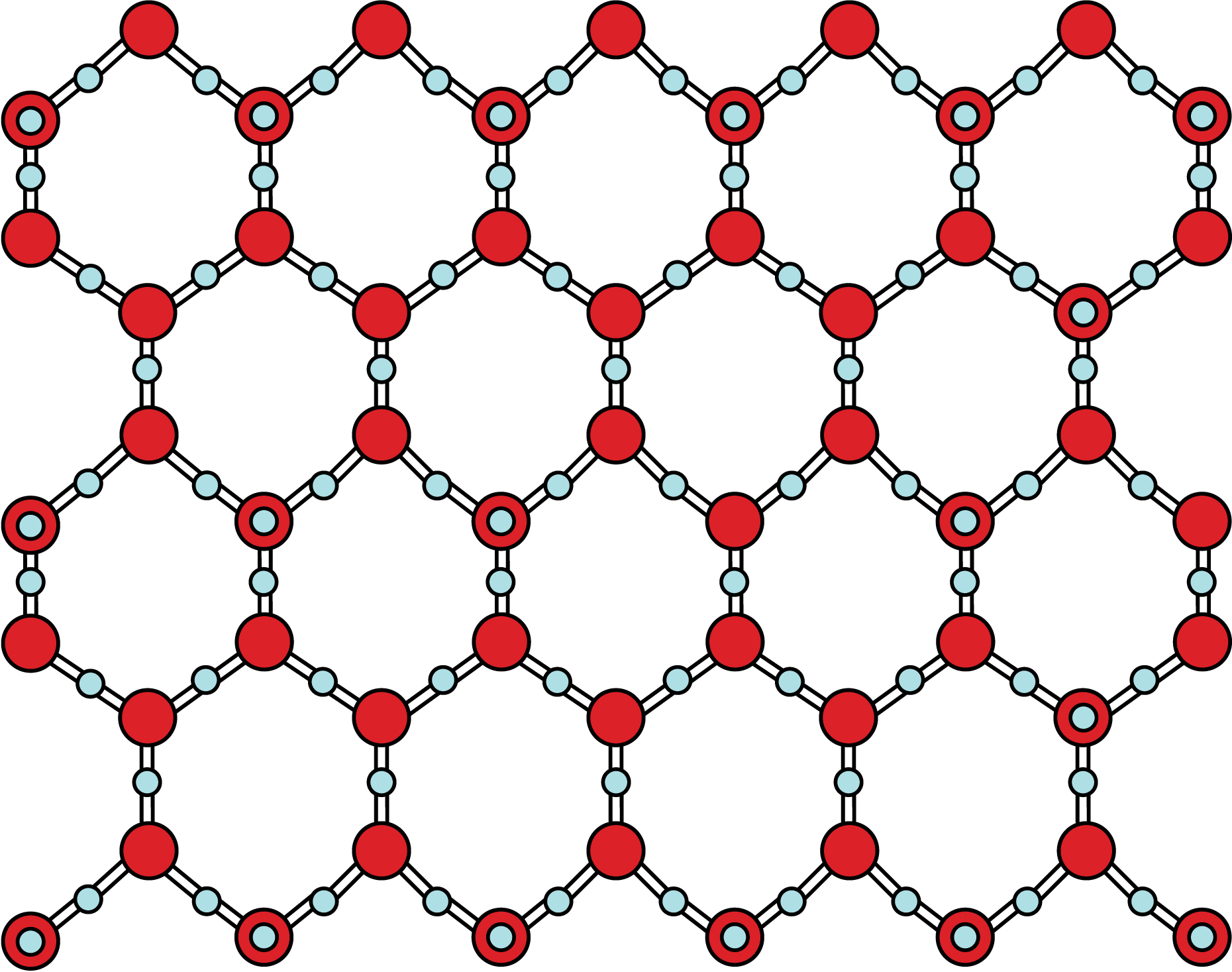 |
| Crystal Structure of Water |
 |
| A Hydrogen Bistrifluoroacetates |
- "Hydrogen bonding" must occur in the molecule (this is sort of assumed if we are talking about symmetric "hydrogen bonding"
- The O···O bond distance must be as small as possible
- The molecule containing the hydrogen bonding should be in a crystal form
 |
| Strong Symmetric "Hydrogen Bond" |
With all of these conditions met, a regular, rather strong, "hydrogen bond" becomes an incredibly powerful symmetrical "hydrogen bond"
No comments:
Post a Comment